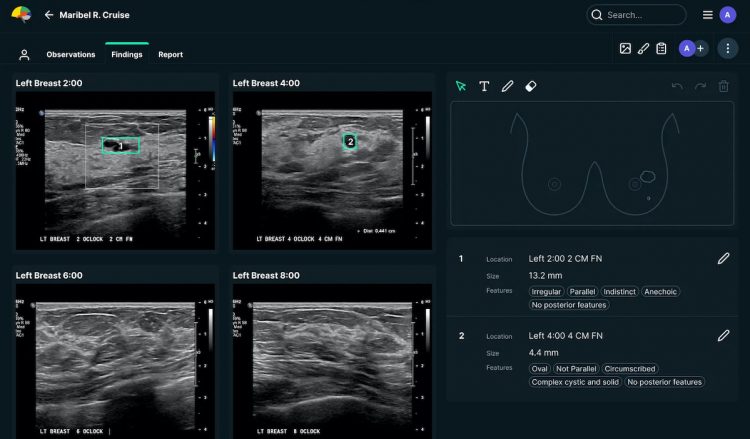
See-Mode Technologies, a medtech startup based in Melbourne, Australia and Singapore, has received the approval of Health Canada for its software product that supports breast and thyroid ultrasound examinations.
Founded in 2017, the company develops AI to better predict the risk of stroke and vascular diseases by improving the analysis of ultrasound images. Its flagship product, Augmented Vascular Analysis (AVA), automatically analyses and produces a report of vascular ultrasound scans.
Over the past two years, See-Mode has also obtained regulatory approvals for its AVA software in the United States, Europe and Australia. The company, which has so far raised $10 million in funding, is backed by prominent venture capital firms, including MassMutual Ventures, Blackbird Ventures, Cocoon Capital, and SGInnovate.
The company has recently expanded the application of its AI software to analyse breast and thyroid ultrasound scans.
HOW IT WORKS
See-Mode’s medical AI software detects lesions in ultrasound images and assigns feature classification to each, in line with the American College of Radiology’s BI-RADS and TI-RADS rating systems. It can also instantly generate worksheets, including classifications and diagrams, and send them to PACS while preliminary impressions are delivered to radiology reporting systems.
In follow-up scans, the solution can automatically highlight changes in lesion characteristics, providing a fast comparison between old and new images.
WHY IT MATTERS
This latest clearance confirms the applicability of See-Mode’s software beyond vascular studies or examinations that it currently supports.
According to Dr Milad Mohammadzade, See-Mode co-founder and director, the reporting of breast and thyroid examinations are usually time-consuming, subjective, and prone to error, especially in the case of multiple lesions.
For thyroid studies, See-Mode can improve the accuracy of biopsies while potentially reducing the conduct of unnecessary tests. For breast ultrasound exams, the software can help detect malignancies that may otherwise go undiscovered or misclassified.
“See-Mode eliminates tedious manual steps in the interpretation and reporting of ultrasound images, improves efficiency, and provides unparalleled consistency of reporting between different members of the radiology team,” Martin Necas, a specialist sonographer at Waikato Hospital and clinical consultant for See-Mode.
MARKET SNAPSHOT
There are at least two more US Food and Drug Administration approvals for AI-powered breast cancer screening solutions in the past two years: the Volpara Imaging Software by New Zealand-based Volpara Health and American AI startup Whiterabbit’s WRDensity Software.
Canada and Singapore-based Medo also recently gained a 510(k) for its ultrasound diagnostic assistant tool, Medo Thyroid, which helps identify and evaluate adult thyroid nodules.
Meanwhile, Shenzhen-listed medical device company Mindray unveiled last year its imaging diagnostic ultrasound system called Resona I9, which can also automatically analyse common lesions in breast and thyroid scans.
Source by www.mobihealthnews.com